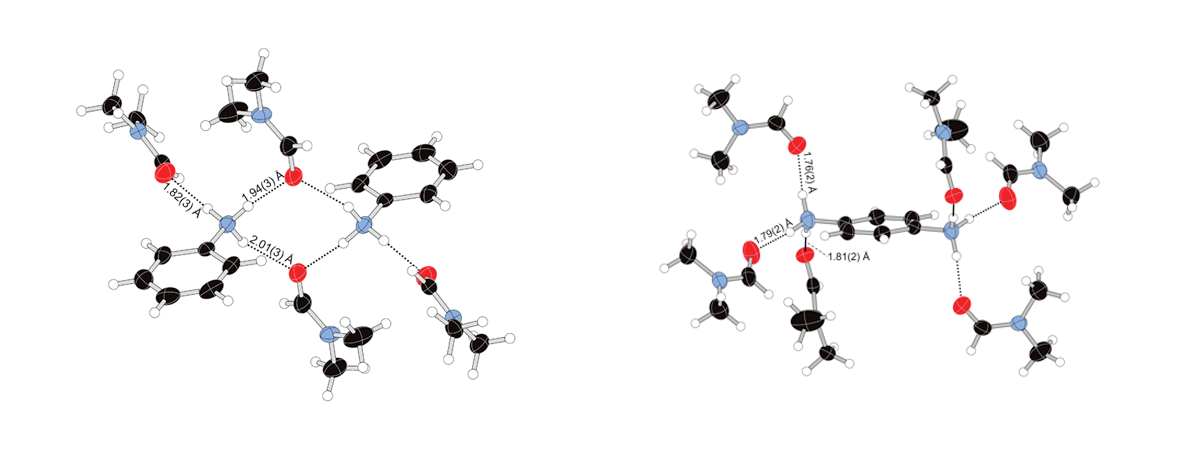
 Hydrogen bonding between DMF and ammonium ions
Hydrogen bonding between DMF and ammonium ions
Crystal structures and hydrogen-bonding analysis of a series of solvated ammonium salts of molybdenum(II) chloride clusters
Abstract
Charge-assisted hydrogen bonding plays a significant role in the crystal structures of solvates of ionic compounds, especially when the cation or cations are primary ammonium salts. We report the crystal structures of four ammonium salts of molybdenum halide cluster solvates where we observe significant hydrogen bonding between the solvent molecules and cations. The crystal structures of bis(anilinium) octa-μ3-chlorido-hexachlorido-octahedro-hexamolybdate N,N-dimethylformamide tetrasolvate, (C6H8N)2[Mo6Cl8Cl6]·4C3H7NO, (I), p-phenylenediammonium octa-μ3-chlorido-hexachlorido-octahedro-hexamolybdate N,N-dimethylformamide hexasolvate, (C6H10N2)[Mo6Cl8Cl6]·6C3H7NO, (II), N,N′-(1,4-phenylene)bis(propan-2-iminium) octa-μ3-chlorido-hexachlorido-octahedro-hexamolybdate acetone trisolvate, (C12H18N2)[Mo6Cl8Cl6]·3C3H6O, (III), and 1,1′-dimethyl-4,4′-bipyridinium octa-μ3-chlorido-hexachlorido-octahedro-hexamolybdate N,N-dimethylformamide tetrasolvate, (C12H14N2)[Mo6Cl8Cl6]·4C3H7NO, (IV), are reported and described. In (I), the anilinium cations and N,N-dimethylformamide (DMF) solvent molecules form a cyclic R42(8) hydrogen-bonded motif centered on a crystallographic inversion center with an additional DMF molecule forming a D(2) interaction. The p-phenylenediammonium cation in (II) forms three D(2) interactions between the three N—H bonds and three independent N,N-dimethylformamide molecules. The dication in (III) is a protonated Schiff base solvated by acetone molecules. Compound (IV) contains a methyl viologen dication with N,N-dimethylformamide molecules forming close contacts with both aromatic and methyl H atoms.