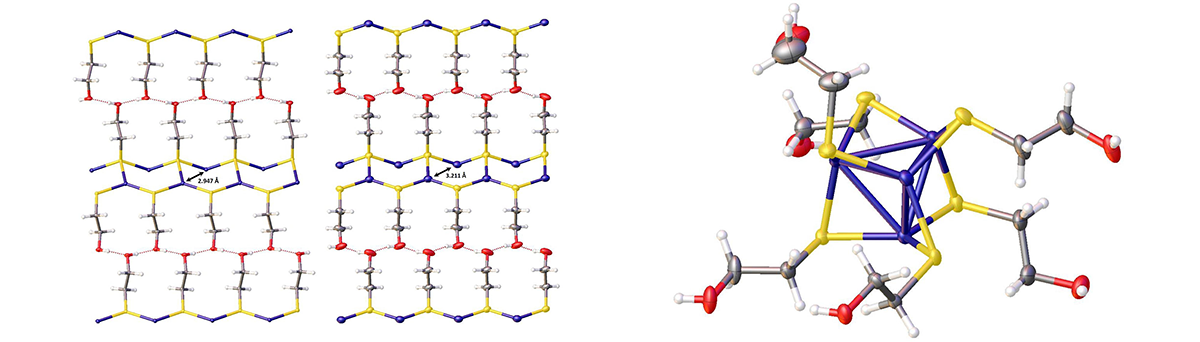
 Copper coordination polymer and copper cluster unit
Copper coordination polymer and copper cluster unit
Crystalline Phase Transitions and Water-Soluble Complexes of Copper(I) 2-Hydroxyethanethiolate
Abstract
The coordination polymer copper(I) 2-hydroxyethanethiolate, (CuSCH2CH2OH)n, though insoluble in all common solvents, dissolved readily in basic aqueous solutions of the thiolate anion (HOCH2CH2S–) of 2-mercaptoethanol to form a single species: the tetranuclear cluster [Cu4(μ-SCH2CH2OH)6]2–. From this solution were grown X-ray quality single crystals of copper(I) 2-hydroxyethanethiolate. This compound underwent a hitherto unknown crystal phase transition at ca. 6 °C, from point group P212121 to Pna21, with noticeable changes in the geometry of the Cu-S layer and in the orientation of the alkylthiolate side chains. When the bulky base tetrabutylammonium hydroxide was employed in the aqueous thiolate solution used to dissolve (CuSCH2CH2OH)n, the water-soluble polynuclear copper(I) complex bis(tetrabutylammonium) hexakis(µ-2-hydroxyethanethiolato) tetracuprate(I), [(C4H9)4N]2[Cu4(μ-SCH2CH2OH)6], could be isolated as X-ray quality crystals. Structural characterization of this complex revealed a tetrahedral arrangement of copper(I) centers with thiolates bridging the edges of the tetrahedra. On standing, this complex degraded to a larger polynuclear Cu(I) sulfide cluster.