 Generation of polyacetylene ionomers via ROMP
Generation of polyacetylene ionomers via ROMP
Kinetic Study of the Ring-Opening Metathesis Copolymerization of Ionic with Nonionic Cyclooctatetraene Derivatives To Yield Polyacetylene Ionomers
Abstract
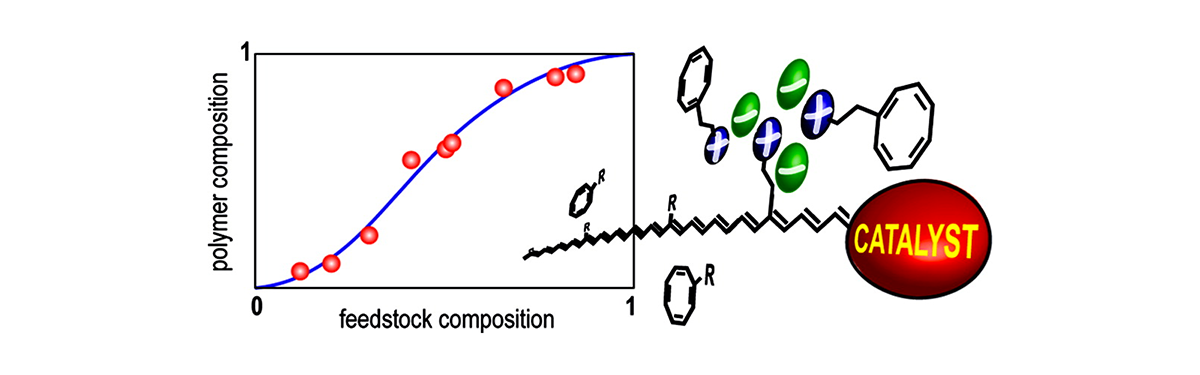
The ion density in ionically functionalized polyacetylenes can be controlled by the ring-opening metathesis copolymerization of functionalized cyclooctatetraene (RCOT) monomers. Studies are reported on the kinetics for copolymerization of anionic (MA, R = −CH2CH2SO3−NMe4+ ) and cationic (MC, R = −CH2CH2NMe3+CF3SO3−) RCOTs with a nonionizable RCOT (MT, R = −SiMe3) initiated by the well-defined tungsten imidoalkylidene catalyst W(CH(o-C6H4OMe))(NC6H5)(OCCH3(CF3)2)2(THF). Twenty separate copolymerizations were studied as a function of the mole fraction of ionic monomer in the feedstock. The monomer consumption was observed to follow zero-order behavior over a range of monomer concentration in the MA−MT system, whereas it was observed to follow first-order behavior in the MC−MT system. The zero-order behavior in the MA−MT system was observed to lead to much less drift in polymer composition than predicted by classic copolymerization theory. The initial rates of monomer conversion were determined and used to calculate the copolymer composition curve. The curves exhibited sigmoidal shapes as described by reactivity ratios rC = 8 ± 3 and rT = 4 ± 2 for the MC−MT system and rA = 2.0 ± 0.3 and rT = 2.3 ± 0.3 for the MA−MT system. In all cases, the rate of initiation was observed to be slow relative to propagation, and the initiation rate for MT was observed to be approximately a factor of 4 greater than for the ionic monomers under similar conditions of monomer and catalyst concentration. The observed trends are explained in terms of ion−ion interactions. These interactions are argued to create different local activities of the ionic monomers in the vicinity of an active catalyst center with ultimate ionic monomer as compared to near either the uninitiated catalyst or an active catalyst center with ultimate nonionic monomer. The largest effects are observed in the MC−MT system where, in addition to the largest reactivity ratios, the ratio of the overall polymerization rate to the initiation rate increases by a factor of 20 in progressing from the homopolymerization of MT to that of MC.