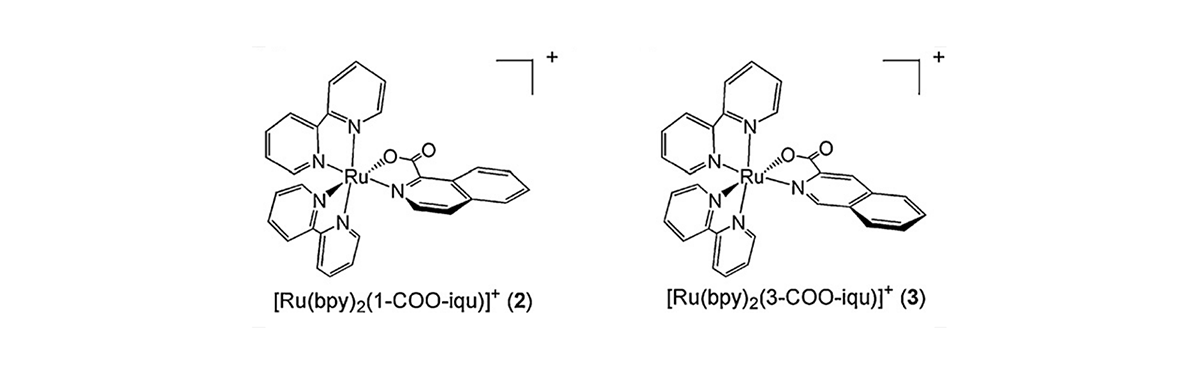
 Molecular structures of compounds 2 and 3
Molecular structures of compounds 2 and 3
Effect of intraligand π-delocalization on the photophysical properties of two new Ru(II) complexes
Abstract
Two new Ru(II) complexes, [Ru(bpy)2(1-COO-iqu)]+ (2; bpy = 2,2′-bipyridine, 1-COO-iqu− = isoquinoline-1-carboxylate) and [Ru(bpy)2(3-COO-iqu)]+ (3; 3-COO-iqu− = isoquinoline-3-carboxylate), were prepared and their crystal structures solved. The ground and excited state properties of 2 and 3 were characterized and compared to those of [Ru(bpy)3]2+ (1). The presence of the oxygen atom in the Ru(II) coordination sphere makes 2 and 3 easier to oxidize than 1. The Ru → bpy MLCT absorption and emission of 2 and 3 are red-shifted relative to that of 1 in CH2Cl2, and the E00 energies were estimated to be 1.89 eV and 1.95 eV from the low temperature emission of 2 and 3, resulting in excited state oxidation potentials of −1.03 V and −1.10 V vs SCE, respectively. In addition to the short-lived emissive 3MLCT state, a long-lived species is observed in the transient absorption of 3 in DMSO (τ = 49 μs) and pyridine (τ = 44 μs), assigned to a solvent-coordinated complex. This intermediate is not observed for 3 in non-polar solvents or for 2. The absence of the solvent coordinated intermediate in 2 is explained by the stronger Ru–O bond afforded by the lower conjugation in that extends onto the carboxylic acid in the 1-COO-iquo−ligand, compared to that in the 3-COO-iqu−ligand in 3. Transient absorption experiments also show that the 3MLCT excited state of 3 is able to reduce methyl viologen.