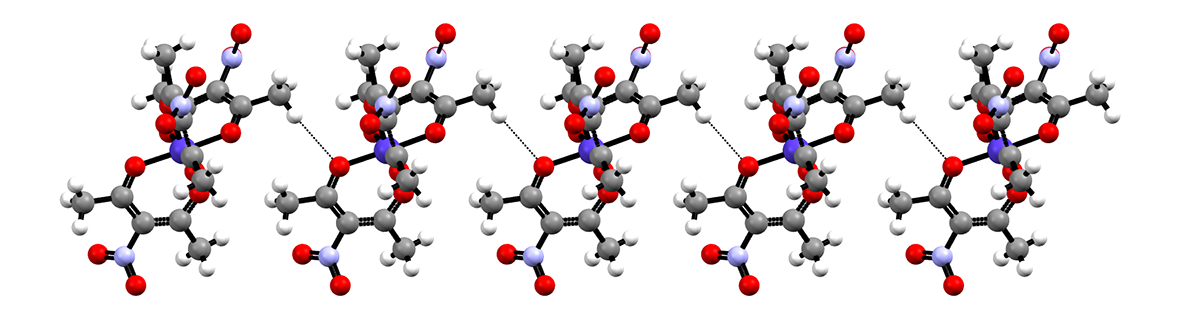
 Hydrogen-bonded chains formed by the cobalt acetylacetonate complex
Hydrogen-bonded chains formed by the cobalt acetylacetonate complex
Tris(3-nitropentane-2,4-dionato-Κ2O,O′)- cobalt(III)
Abstract
The structure of the title compound, [Co(C5H6NO4)3], consists of a CoIII ion octahedrally coordinated by three bidentate 3- nitropentane-2,4-dionate ligands. The complex was prepared via the nitration of tris(2,4-pentanedionato- 2O,O0)cobalt(III) with a solution of copper(II) nitrate in glacial acetic acid. The central C atom and the nitro group of one 3-nitropentane-2,4- dionate ligand are disordered over two positions with an occupancy ratio of 0.848 (4):0.152 (4). A second nitro group is also disordered over two orientations with an occupancy ratio of 0.892 (7):0.108 (7). Two of the ligand methyl groups form C—H O interactions with two different nitro groups to form chains running along the c axis. Additional C—H O interactions are found between ligand methyl groups and the cobalt-bound O atoms, also resulting in the formation of chains along the c axis.