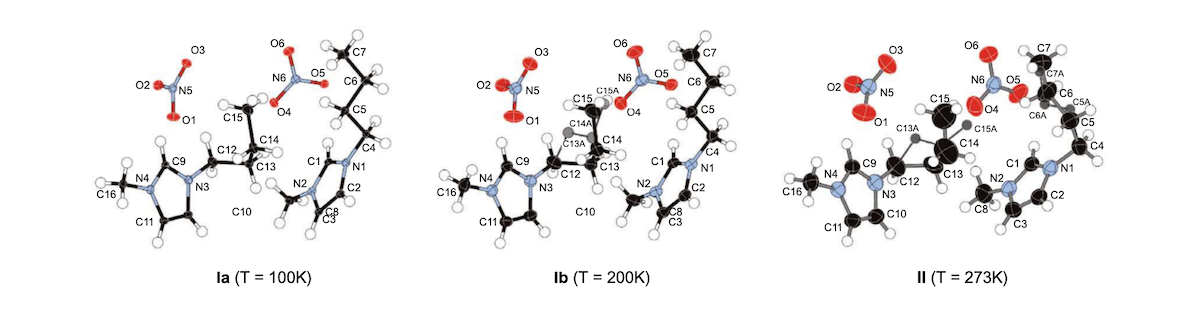
 Structure of the BMIM-nitrate ionic liquid at three different temperatures.
Structure of the BMIM-nitrate ionic liquid at three different temperatures.
Structural and Conformational Analysis of 1-Butyl-3-methylimidazolium Nitrate
Abstract
The crystal structures of two polymorphic forms of 1-butyl-3-methylimidazolium nitrate are reported. The form observed at 100 K and 200 K (Ia and Ib) crystallizes in the P-1 space group and contains two independent imidazolium cations and nitrate anions. At 100 K the butyl chain of one cation adopts a TT (trans-trans) conformation and the other cation adopts a G’G’ (gauche-gauche) conformation. At 200 K, the G’G’ chain is disordered with about twelve percent of the GT conformation present. A different polymorph (Form II, also crystallizing in the P-1 space group with two independent ion pairs) is present at 273 K displaying significant disorder in the butyl chains with a mixture of G’T, G’G’, and GT conformers. Raman spectra were collected on samples between 100 and 350 K and show changes in band frequencies and intensities consistent with conversion between different butyl chain conformations. Hydrogen bond interactions are present between cation C-H’s and oxygen atoms of the nitrate ions, with significant lengthening observed for three of the six close contacts (and formation of one new contact) upon conversion to the higher-temperature form. The structural details revealed in this study shed light on the intermolecular forces and the conformational changes that accompany phase changes in 1-butyl-3-methylimidazolium nitrate.