 |
 |
 | ||
In the organic portion of this course, we learned that an amide can be thought of as a derivative of a carboxylic acid and an amine. Under most conditions an acid and a base combine to give a salt, however, amino acids can be linked together using enzymes to form a peptide bond. Short sequences of amino acids linked together in this way are called peptides.
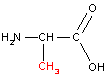
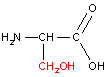
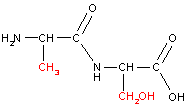
Let us consider linking together two amino acids, alanine and serine:
 |
 |
 | ||
or, combined in the reverse order...
 |
 |
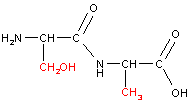 | ||
Notice how with the same two amino acids we can form two different dipeptides. An example of a tripeptide (three amino acids linked by peptide bonds) is shown below. Proteins are polypeptide chains (polymers of amino acids).
Every peptide (and protein chain) has two ends, the N-terminus and the C-terminus. The individual amino acids are linked together by amide linkages called peptide bonds. The repeating -N-C-C- unit (shown below in blue) is called the backbone and the R-groups (in red) are called sidechains.
alanine serine glycine
Figure 1. A typical tripeptide consisting of an ala-ser-gly (or ASG) sequence of amino acids.