Structure of Amino Acids
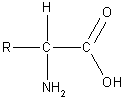 Proteins are polymers - long chain molecules made up of many repeating
units called amino acids. Amino acids all have similar structures
(shown on the right) with two functional groups, an amino (-NH2)
group and a carboxylic acid (-COOH) group. The only difference between
different amino acids is the R group.
Proteins are polymers - long chain molecules made up of many repeating
units called amino acids. Amino acids all have similar structures
(shown on the right) with two functional groups, an amino (-NH2)
group and a carboxylic acid (-COOH) group. The only difference between
different amino acids is the R group.
There are twenty naturally occurring amino acids which are classified into four groups according to the properties of the -R group. These four groups, along with example amino acids, are shown in the table below.
| |
|
|
| |
|
 |
| |
|
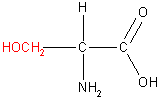 |
| |
|
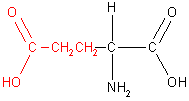 |
| |
|
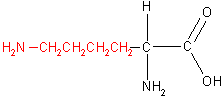 |
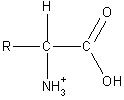
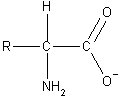
Acid/Base Properties of Amino Acids
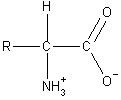 Amino acids are unique in that they possess both an acidic and a basic
functional group on the same molecule. In fact, amino acids do not exist
in the form shown in the above figures. The carboxylic acid donates its
proton to the amino group and a zwitterion is formed (shown on the
right).
Amino acids are unique in that they possess both an acidic and a basic
functional group on the same molecule. In fact, amino acids do not exist
in the form shown in the above figures. The carboxylic acid donates its
proton to the amino group and a zwitterion is formed (shown on the
right).
As you might imagine, the actual form of the amino acid will depend on the pH of its environment. At low pH both the acid and amino groups will be protonated and at high pH both the acid and amino groups will be deprotonated. The pH at which the zwitterionic form predominates is called the isoelectric point. This is summarized in the figure below.