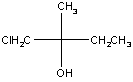
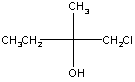
Example 1: 1-chloro-2-methyl-2-butanol 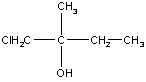
This molecule is chiral because the central carbon (carbon 2) is bonded to four different groups. Fischer projections and three-dimensional models of both enantiomers of this molecule are shown in the table below.
| Molecule |
Mirror Image | |
| Fischer Projection |
|
|
| 3D Picture |
|
|
Example 2: 2-bromo-2-chlorocyclopentanone 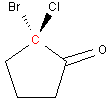
Cyclic molecules can also be chiral. In this case it is harder to see that there are four different "groups" on the carbon 2 (in red), but the carbons are different depending on which direction you go around the ring. Question: Would 1-bromo-1-chlorocyclopentane be chiral?
|
|
|
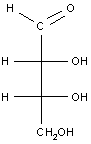
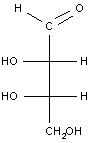
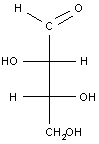
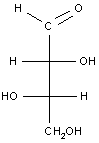
Example 3: A simple carbohydrate (an aldotetrose)
The last example is a relatively simple carbohydrate with two chiral carbon atoms. There should be a total of 22 = 4 optical isomers, or two pairs of enantiomers, which are shown in the table below. For carbohydrates, as we will see in class, the two enantiomers are identified as D or L. Each set of enantiomers is given a separate name.
| D-configuration |
L-configuration | |
| erythrose |
|
|
| threose |
|
|
Verify that the following statements are true.
- D-threose and L-threose are enantiomers
- D-erythrose and D-threose are diastereomers
- L-threose and D-erythrose are diastereomers