Up to this point we have only considered molecules with one chiral carbon. In many biomolecules, specifically carbohydrates, there may be two, three, four, or more chiral carbons in one molecule.
For each chiral carbon in a molecule, there are two possible arrangements around that carbon atom. For a molecule with two chiral carbons, there will therefore be a total of:
(2 centers) x (2 arrangements) = 4 optical isomers.
This can generalized for any number of chiral carbons:
# of optical isomers = 2n (where n = # of chiral carbons)
Let us now consider a hypothetical molecule (shown in Figure 1) with two chiral carbons. There will be a total of four optical isomers, or two pairs of enantiomers. The relationships between the four optical isomers are shown in Figure 1.
| |
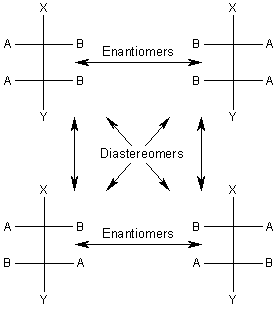 |
From this diagram we see that any two molecules that are optical isomers but not enantiomers are called diastereomers.