|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
 |
|
|
|
|
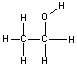
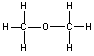
Shown in the table below are two molecules which are structural isomers of eachother. Notice that they have the same chemical formula, but different structural formulas (and therefore different structures and properties.)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
 |
|
|
|
|
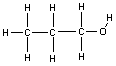
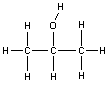
Structural isomerism may also be more subtle, involving only a slight difference in the way the atoms are connected, rather than completely different molecules. An example of this is the difference between 1-propanol and 2-propanol (also known as isopropanol or rubbing alcohol). The only difference between these two molecules is the position at which the -OH (alcohol) group is attached. These two molecules are structural isomers.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
 |