A second type of isomerism, geometrical isomerism, is found whenever an organic molecule contains a bond around which rotation is restricted. The two cases where this is found is in cyclic (ring) molecules and in alkenes. One example is shown below for cyclohexane, but the same principles apply to isomerism in alkenes as well.
The general rules for determining if geometrical isomerism is possible for a molecule are as follows:
- Does the molecule contain a bond around which rotation is restricted (double-bond or cylic system)? If not then geometrical isomers are not possible.
- Are there two carbon atoms (within the ring or connected by a double bond) that are both bonded to two different groups? If not then geometrical isomers are not possible.
Geometrical isomers are named as either cis (same side) or trans (opposite sides) isomers. Study the example below.
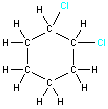
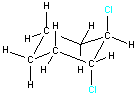
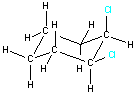
Example 1: 1,2-dichlorocyclohexane
| For some molecules, the two-dimensional representation may not communicate all the information about the three-dimensional structure. Consider the two structures shown on the right. Both represent 1,2-dichlorocyclohexane and both show the connectivity of the atoms within the molecule. However, they do not give information about the three-dimensional arrangement of the two chlorine atoms. The two possible arrangements (cis and trans) are shown in the table below. Notice how the three-dimensional structure can be represented in a two-dimensional drawing. |
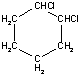
|