|
|
|
|
|
|
 |
|
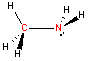
Nitrogen - sp3 hybridization
The nitrogen atom also hybridizes in the sp2 arrangement, but differs from carbon in that there is a "lone pair" of electron left on the nitrogen that does not participate in the bonding. The geometry about nitrogen with three bonded ligands is therefore trigonal pyramidal.
|
|
|
|
|
|
 |
|
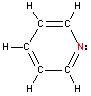
Nitrogen -sp2 hybridization
Nitrogen will also hybridize sp2 when there are only two atoms bonded to the nitrogen (one single and one double bond). Just as for sp3 nitrogen, a pair of electrons is left on the nitrogen as a lone pair. The resulting geometry is bent with a bond angle of 120 degrees.
|
|
 |
|
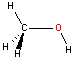
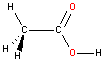
Oxygen - sp3hybridization
Oxygen bonded to two atoms also hybridizes as sp3. There are two lone pairs of electrons located on the oxygen atom (not shown) and the resulting geometry is bent with a bond angle ~109 degrees. Note that in acetic acid one of the oxygen atoms is bonded to only one atom. We therefore do not have to consider the geometry (or hybridization) around that particular atom.
|
|
|
|
|
|
 |
|
|
|
 |
|
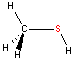
Sulfur - sp3 hybridization
The geometry of sulfur compounds is essentially the same as for oxygen compounds with sp3 hybridization found with two atoms bonded to sulfur.
|
|
|
|
|
|
 |