|
|
 |
|
|
|
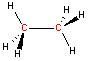 |
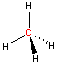
Carbon - sp3 hybridization
When carbon is bonded to four other atoms (with no lone electron pairs), the hybridization is sp3 and the arrangement is tetrahedral. Notice the tetrahedral arrangement of atoms around carbon in the two and three-dimensional representations of methane and ethane shown below. (Click and "drag" within the black box to rotate the three-dimensional structure.)
|
|
 |
|
|
|
 |
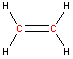
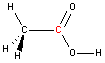
Carbon - sp2 hybridization
A carbon atom bound to three atoms (two single bonds, one double bond) is sp2 hybridized and forms a flat trigonal or triangular arrangement with 120° angles between bonds. Notice that acetic acid contains one sp2 carbon atom and one sp3 carbon atom.
|
|
 |
|
|
|
 |
Carbon - sp hybridization
The third possible arrangement for carbon is sp hybridization which occurs when carbon is bound to two other atoms (two double bonds or one single + one triple bond). This hybridization results in a linear arrangement with an angle of 180° between bonds.
|
|
|
|
|
|
|