
THE SUN
PHYSICAL FEATURES
Diameter 100 x Earth--larger than orbit of moon
(from known distance, and apparent size)
Volume: 1 million x Earth
Mass: 300 thousand x Earth (from orbit of planets: Newton)
Density: 300 thousand/1 million= 0.3 x Earth
(comparable to Jovians)
Temperature of visible surface: 5800 K (10,000° F)
(from black body spectrum)
Composition: mostly hydrogen, 9% helium; traces of other elements
(from dark lines in solar spectrum, and density) --
note similarity to Jupiter
Energy output: 4x1026 Watts (power=energy per unit
time) produced by nuclear fusion
SOLAR ENERGY
All the energy used on Earth (with the exception of nuclear power, available
since 1940's) comes directly or indirectly from the Sun. Examples:
- fossil fuels come from plants that store energy by photosynthesis
- wind to power windmills comes from convection driven by solar heat
- waterfalls powered by water evaporated by solar heat deposited as rain
in the mountains
Power per unit area=1400 W/m2 (solar
constant)
every m2 at distance of Earth receives same amount of
power -- total power, or luminosity,
= power per area x total area
INTERNAL STRUCTURE
Deduced from models of the solar interior. Models must:
- agree with all observable properties of the Sun
- obey known laws of physics, as applied to the conditions in the interior
of the Sun.
Additional information can be deduced from solar
oscillations
- vibrations of the Sun's surface, detected by their Doppler shift
- due to waves propagating through the Sun, like seismic waves through
the Earth: helioseismology
- different waves have different frequencies. It's a little like trying
to determine the shape of a bell by listening to the sound of its ringing!
Generally, the temperature, pressure and density of the Sun drop off
as we move out from the center. Computer models must calculate the values
of these parameters, as well as others, such as chemical composition, etc.
PARTS OF THE SUN
- Core: region of nuclear fusion (source
of power); temperature, 15 million K (27 million oF); high pressure
(due to weight of gas above)
- radiation zone: ("interior")
energy travels through this region in form
of EM radiation, occasionally scattering from nuclei.
- convection zone: in this region energy
(heat) is carried by convection.
It can take 100 thousand years for heat from the core to escape this zone.
- photosphere: where density of sun drops
to the point that the gas is transparent to radiation. From this point,
heat is
carried away from the Sun as black body radiation (8 minutes to Earth).
The photosphere is essentially the top layer of the convection zone.
-
granules, 1000 km across, visible in photosphere,
are the tops of convection cells, cf. bands on Jupiter.
(velocity determined by Doppler shift)
Note. There are no sharp surfaces in the Sun, it's all gas
SOLAR ATMOSPHERE (layers above photosphere)
-
chromosphere: gas too thin to glow brightly,
but visible during solar eclipse. Characteristic red color due to emission
line of hydrogen.
- spicules, thin jets
of matter thrown out from the photosphere.
-
Cooler upper parts of chromosphere contribute dark
lines to solar spectrum
corona: thin hot gas above chromosphere.
-
Temperature deduced from spectral lines of elements that have lost
some of their electrons; emission in X-ray region.
Cause of high temperature not known.
-
Upper regions of corona are source of solar
wind:
far enough from Sun that the high temperature
allows atmosphere to escape from Sun's gravity.
SOLAR MAGNETISM AND ACTIVE REGIONS OF THE SUN
Sunspots
- dark, cooler regions on the photosphere, first observed by Galileo.
- Usually occur in pairs.
- Frequency of occurrence varies with time: maximum approximately every
11 years (solar sunspot cycle)
- associated with magnetic field (affects spectral lines); possibly
caused by interaction of magnetic field with differential rotation
- curiously absent from 1645-1715 (Maunder minimum)
Prominences
- loops or sheets of gas; tend to follow magnetic field lines near sunspot
pairs.
- last for hours, days, weeks; can be larger than Earth
- cause unknown.
Flares
- like prominences, but so energetic that material is ejected from the
Sun
- temperatures up to 100 million K
- flares and prominences more common near sunspot maxima
Sun's brightness varies by a few parts in a thousand near sunspot maxima
-- climate connection
HISTORY OF THEORIES ABOUT THE ORIGIN OF THE SUN'S ENERGY OUTPUT
- Anaxagoras (500-428 BC) thought the Sun was a large, hot rock.
- If the Sun produced power by combustion, it could last a few thousand
years.
- Kelvin and Helmholtz (19th century)
propose Sun's energy comes from
gravitational contraction. This would give the Sun
a lifetime of 100 million years. (There probably exist bodies that give
off heat from this process, called brown dwarfs)
- By the end of the 19th century, geological evidence showed that the
Earth was much older; and with the discovery of radioactivity, the age
of the Earth was determined to be 4.5 billion years. A new
explanation was required.
NUCLEAR FUSION
What is a nucleus?
- Atoms are composed of electrons orbiting nuclei, held together by electrical
forces. (19th C)
- Nuclei (10-15 m) are very small compared to the size of
atoms (10-10 m). Discovered by Ernest Rutherford around 1900.
- Nuclei are composed of protons and neutrons. (1930's)
Nuclear fusion is a reaction between nuclei in which smaller nuclei
join to form a larger nucleus.
The mass of the total products of the reaction is less than the
original mass. Where does the mass go? Mass is not conserved: it can be
converted into energy: E=mc2 (Einstein, 1905).
- c, the speed of light, is very large, so a small
amount of mass yields a lot of energy
- to produce the amount of power emitted by the Sun, 600 million tons
of hydrogen must be combined to form helium per second: a lot of mass,
but small compared to the Sun
- at this rate, the Sun has enough fuel to last another 5 billion years
NUCLEAR POWER
Requirements for a fusion reaction are high temperature
and high density: this has been achieved on
Earth in the hydrogen bomb, but never in a controlled reactor (beyond very
preliminary tests)
Fission is a nuclear reaction in which
large, heavy nuclei such as those of uranium or plutonium are split into
smaller nuclei plus byproducts. Again, the total mass of the products is
less than that of the original nuclei, and the mass difference is converted
into energy. Nuclear fission occurs in atom bombs, and in
controlled nuclear reactors.
Problems with nuclear fission as a source of power:
- the supply of fuel is limited.
- byproducts are extremely dangerous
- the technology is expensive
- the power plants have limited lifetimes because of the radiation emitted
in the reactor
Controlled fusion, if achieved,
would solve the first two problems but probably not the others
THE PROTON-PROTON REACTION AND THE SOLAR NEUTRINO PROBLEM
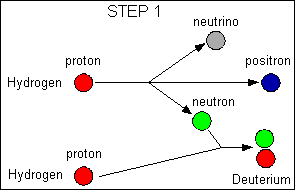
A positron is
an antimatter electron; a neutrino
is
a particle with no charge and almost no mass
that hardly interacts with anything
|
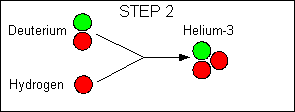
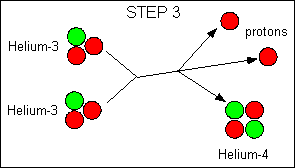
|
Net reaction:
4 hydrogen - > 1 Helium + 2 positrons + 2 neutrinos + energy
The main reaction in the Sun is the proton-proton reaction; some stars
also use another reaction called the carbon cycle, but the net result is
the same
Solar neutrino problem.
Neutrinos can pass right through the Sun, and so it should be possible
to detect the neutrinos coming from the fusion reaction at the core of
the Sun. (Neutrinos turn chlorine nuclei to argon, or gallium to germanium.)
The results are 1/3 to 1/2 the predicted value.
Possible explanations:
1. models of the solar interior are incorrect (e.g. perhaps we haven't
calculated the temperature of the core correctly)
2. our understanding of the physics of neutrinos is incorrect
 Return to table of contents.
Return to table of contents.
 Previous section
Previous section
 Next section
Next section
your instructor
Copyright © 1996 M. S. Pettersen
Permission is granted to make copies for individual use, not for redistribution.
This document was last updated July 3, 1999.




