

WAVES
Examples: ocean waves; sound, light. A wave is usually a disturbance in a medium (water, air, etc.)
Characteristics of a wave:
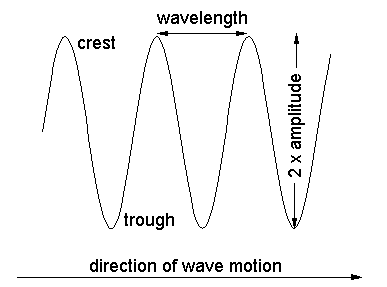
frequency is how frequently a crest
washes over you. Wavelength times
frequency = velocity of wave
(![]() f = c), so you can specify either
f = c), so you can specify either
ELECTROMAGNETIC WAVES or EM RADIATION
radio, infrared, visible light, ultraviolet, X-ray, gamma rayOnly visible light, most radio waves and some infrared get through Earth's atmosphere
red, orange, yellow, green, blue, indigo, violet
BLACK BODY RADIATION
Key features:
Temperature scales:
| Kelvin | Centigrade | Fahrenheit | |
| absolute zero | 0 K | -273 °C | -459 °F |
| ice melts | 273 K | 0 °C | 32 °F |
| body temperature | 310 K | 37 °C | 98.6 °F |
| water boils | 373 K | 100 °C | 212 °F |
Applications: measure temperature of distant bodies; 3 K background radiation
SPECTRAL LINES
Some bodies instead of emitting at all frequencies emit only at certain discrete frequencies called bright or emission lines
![]() can be used to identify elements, or chemical compounds
can be used to identify elements, or chemical compounds
Applications: discovery of Helium in sun (1868; found on earth in 1895); identification of composition of bodies at a distance (spectroscopy)
DOPPLER EFFECT
radiation from receding bodies is shifted to longer wavelengths (red shift); radiation from approaching bodies is shifted to shorter wavelengths (blue shift)
velocity towards or away is radial; across the field of view, transverse. Only radial velocity contributes to Doppler shift.
Doppler shift can be used to measure radial velocity.
KIRCHHOFF'S LAWS
1. A luminous solid or liquid (or in fact a sufficiently dense gas) emits light of all wavelengths and so produces a continuous spectrum of radiation: black body
2. A low density hot gas emits light whose spectrum consists of a series of discrete bright emission lines. These lines are characteristic of the chemical composition of the gas.
3. A cool, thin gas absorbs certain wavelengths from a continuous spectrum, leaving discrete dark absorption (or Fraunhofer) lines in their place, superposed on the continuous spectrum. Once again, these lines are characteristic of the composition of the intervening gas and occur at precisely the same wavelengths as the emission lines produced by the gas at higher temperatures.
your instructor