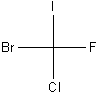
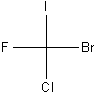
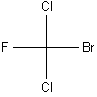
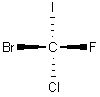It is difficult to convey the three-dimensional structures of complex molecules in a two-dimensional drawing, especially if the molecule contains one or more chiral carbons. Chemists therefore use what is called a Fischer projection (named for German chemist Emil Fischer) to illustrate chemical structures.
In a Fischer projection, the molecule is always oriented such that the four bonds appear to be at 90 degrees with the two bonds coming out of the plane of the paper in the horizontal position and the two bonds going in to the plane of the paper in the vertical position. This is illustrated for CFClBrI below.
| Three illustrations of CFClBrI | ||
| "Wedge" Drawing |
Rotatable Picture |
Fischer Projection |
|
|
|
|
| (Legend: purple = I; yellow = F; green = Cl; red = Br) | ||
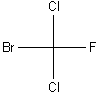
Notice how easy it is to see the chiral carbon in this molecule. It is also a straightforward task to draw its mirror image (shown below).
| Original Molecule |
Mirror Image |
|
|
|
When checking to see if a set of mirror images (drawn as Fischer projections) are superimposable, we are only allowed to:
- move the molecule, and
- rotate the molecule (in the plane of the paper) by 180 degrees
It is clear that the above two molecules are nonsuperimposable mirror images.
Now let's look at a molecule that is not chiral.
| Non-chiral Molecule |
Mirror Image |
|
|
|
The above two molecules cannot be superimposed by simply moving the second molecule over top of the first. By rotating either molecule by 180 degrees, however, the two molecules can be superimposed. They are therefore the same molecule and it is not a chiral molecule.